PREVIOUS
Approval of two gene therapies - USA
December 18 , 2023
343 days
460
0
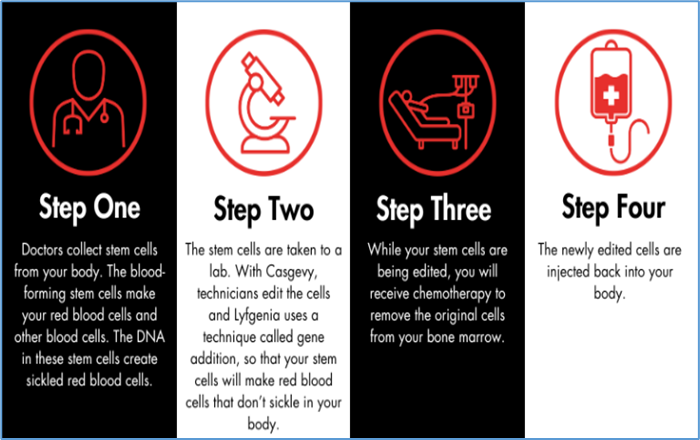
- The U.S. FDA has approved two gene therapies - Casgevy and Lyfgenia - to treat sickle cell disease in patients over 12.
- Its decision on approving Casgevy gene therapy for treating beta thalassemia is expected by March 2024.
- These decisions mark the beginning of gene therapy using the CRISPR-Cas9 tool to treat diseases.
Leave a Reply
Your Comment is awaiting moderation.


