PREVIOUS
Drugs and Clinical Trial Rules, 2019
March 29 , 2019
2472 days
1433
0
- The Union Ministry for Health and Family Welfare has notified the Drugs and Clinical Trials Rules with an aim to promote clinical research in the country.
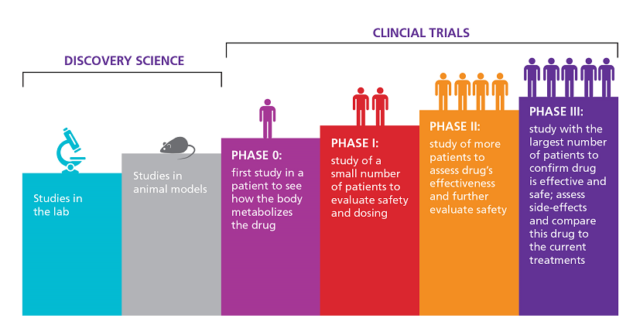
- The new rules will ensure patient safety and the ethics committee will monitor the trials and decide on the amount of compensation in cases of adverse events.
- Drug Controller General of India (DCGI) has waived off the clinical trial for the drugs approved and marketed in the European Union, the UK, Australia, Canada, Japan and the US.
- Clinical trial is a research study that determines whether a new drug is both safe and effective for humans.
Leave a Reply
Your Comment is awaiting moderation.


