PREVIOUS
Drugs and Cosmetics Rules revision – 2025
January 16 , 2025
101 days
241
0
- The Union Health Ministry revised the date of implementation of the revised standards of Schedule M of the Drugs and Cosmetics Act-1945.
- It aims to give the pharmaceutical manufacturing industry 12 more months to comply.
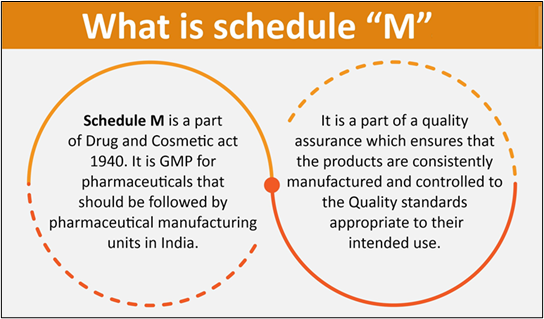
- Schedule M is a part of the Drugs and Cosmetics Act of 1945 that outlines Good Manufacturing Practices (GMP) for pharmaceutical products in India.
- It also covers the requirements for premises, plants, and equipment used in the manufacturing process.
- There are around 10,500 manufacturing units in the country, of which around 8,500 falls under the MSME category.
Leave a Reply
Your Comment is awaiting moderation.


