PREVIOUS
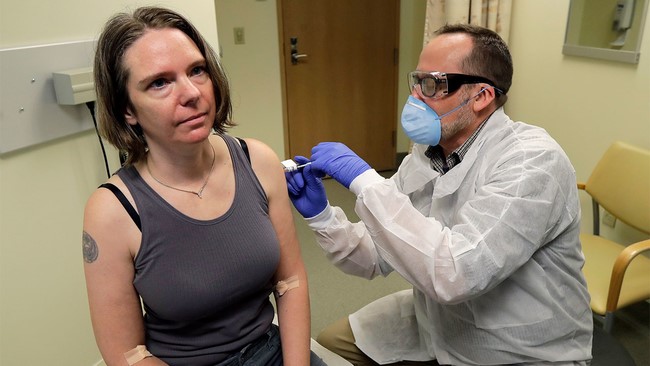
First COVID -19 vaccine trial
March 19 , 2020
1715 days
1076
0
- The United States of America began the first human trial evaluating COVID-19 vaccine.
- The vaccine was developed by a private firm called Moderna.
- During the trial phase, it is to be studied if the vaccines are safe and whether they stimulate the immune system of humans to create antibodies to stop COVID-19.
- The vaccine has been synthesized from a stretch of RNA embedded in a lipid nano particle.
- India contributes only 2.7% of global clinical trials.
- On the other hand, the US contributes 47%, followed by EU of 18%, Japan of 11%.
- In order to improve the situation, India should take active part in platforms like ICTRP of the World Health Organization.
- The International Clinical Trials Registry Platform (ICTRP) of WHO is a mission aimed to make sure a complete view of research is accessible to those who are involved in health care trials.
Leave a Reply
Your Comment is awaiting moderation.


