PREVIOUS
New Alzheimer’s drug
June 19 , 2024
158 days
289
0
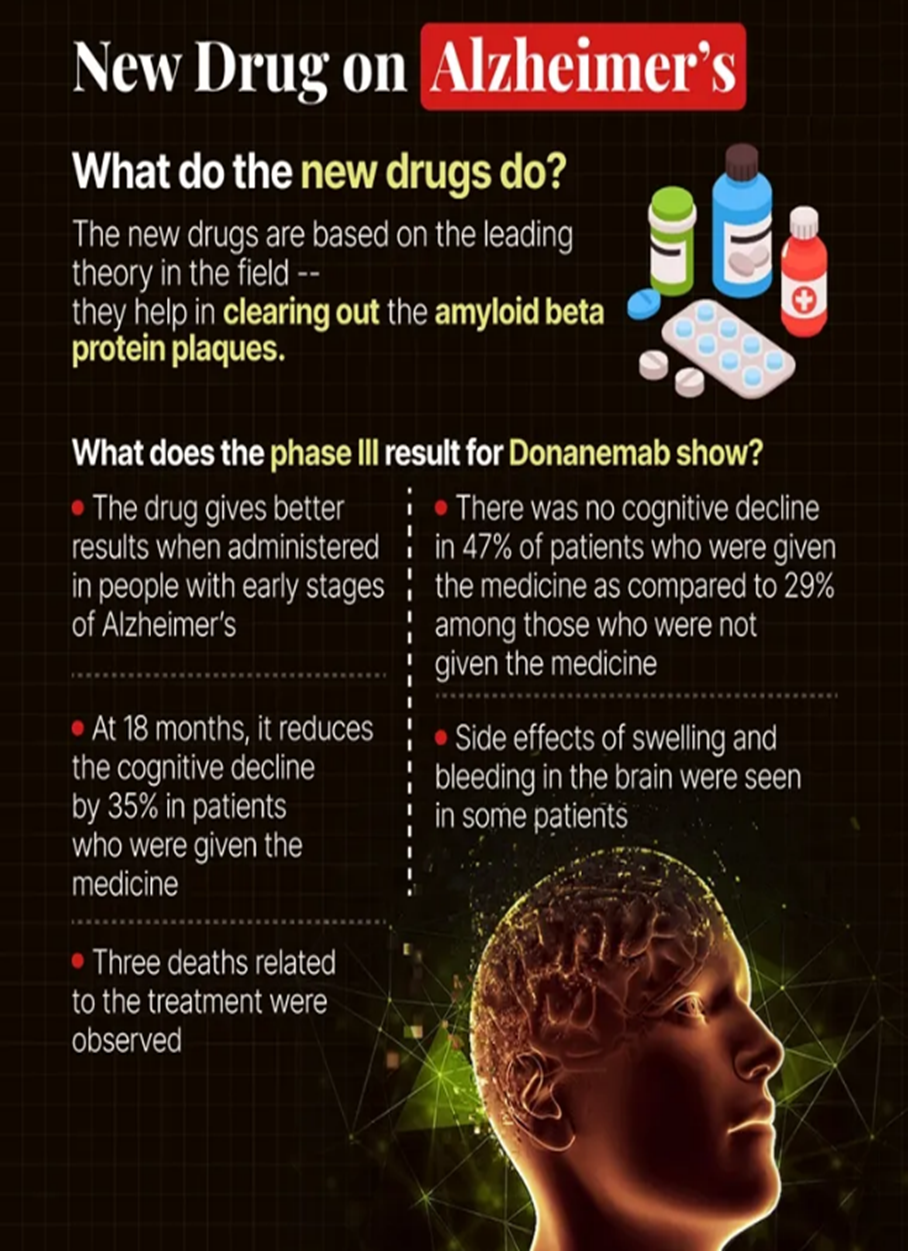
- Independent advisers committee to the US Food and Drug Administration (FDA) gave nod to the new Alzheimer’s drug - Donanemab.
- Donanemab is a monoclonal antibody that targets amyloid beta protein plaques in the brain.
- Though the therapy slows down the progression of the disease, it does not ultimately treat the disease.
- Alzheimer’s disease has multiple modalities and we would need various therapies.
Leave a Reply
Your Comment is awaiting moderation.


