PREVIOUS
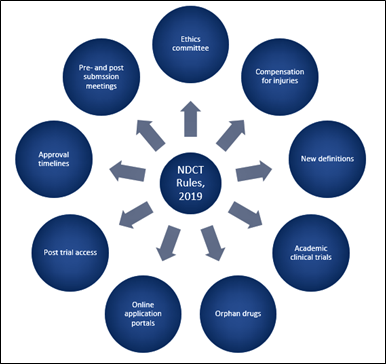
New Drugs and Clinical Trial (NDCT) Rules, 2019
October 24 , 2024
143 days
360
0
- The Drugs Technical Advisory Board (DTAB) has recommended the inclusion of all antibiotics in the definition of new drugs in the New Drugs and Clinical Trial (NDCT) Rules, 2019.
- It aims to curbing the growing anti-microbial resistance which is now recognised as a public health threat globally.
- The board is also looking at amending the labelling requirements under the Drugs Rules, 1945 and adding a blue strip or box for antimicrobial products.
- It has recommended that no antimicrobials should be sold by the traders to the non-pharmaceutical industries who do not hold requisite licences.
- DTAB is the highest statutory decision-making body on technical matters related to drugs in the country.
Leave a Reply
Your Comment is awaiting moderation.


