PREVIOUS
Plasma Therapy
April 13 , 2020
1690 days
992
0
- The Indian Council of Medical Research (ICMR) approved clinical trial protocol to be administered for critically ill COVID-19 patients for conducting convalescent plasma therapy for the treatment of critically ill COVID-19 patients.
- Kerala gets nod for trial of plasma therapy.
- The ICMR has given its nod to the state government for the first-of-its-kind project, initiated by the prestigious Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST) in Kerala.
- Drugs Controller General’s approval and institutional ethics committee approval would have to be there before the treatment can be administered.
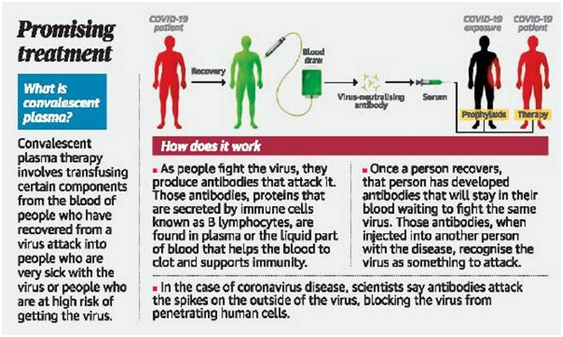
- The therapy takes antibodies from the blood of a person who has recovered from a virus.
- It transfuses those antibodies into a person sick with that virus.
- Those antibodies that are secreted by immune cells known as B lymphocytes, are found in plasma, or the liquid part of blood that helps the blood to clot when needed and supports immunity.
- Once a person has had the virus and recovered, that person has developed antibodies that will stay in their blood waiting to fight the same virus should it return.
- Those antibodies, when injected into another person with the disease, recognize the virus as something to attack.
- The scientists say antibodies attack the spikes on the outside of the virus, blocking the virus from penetrating human cells.
- Convalescent plasma is also known as passive antibody therapy, meaning that while it can immediately provide a person with antibodies to fight a virus, those antibodies only last a short period of time in the recipient’s body.
Leave a Reply
Your Comment is awaiting moderation.


