PREVIOUS
Updated Manufacturing Practices for Drug Makers
January 14 , 2024
395 days
441
0
- The Union Health Ministry notified revised rules under Schedule M of the Drugs and Cosmetics Rules, 1945.
- It was aimed at ensuring robust quality control for pharma and biopharmaceutical products.
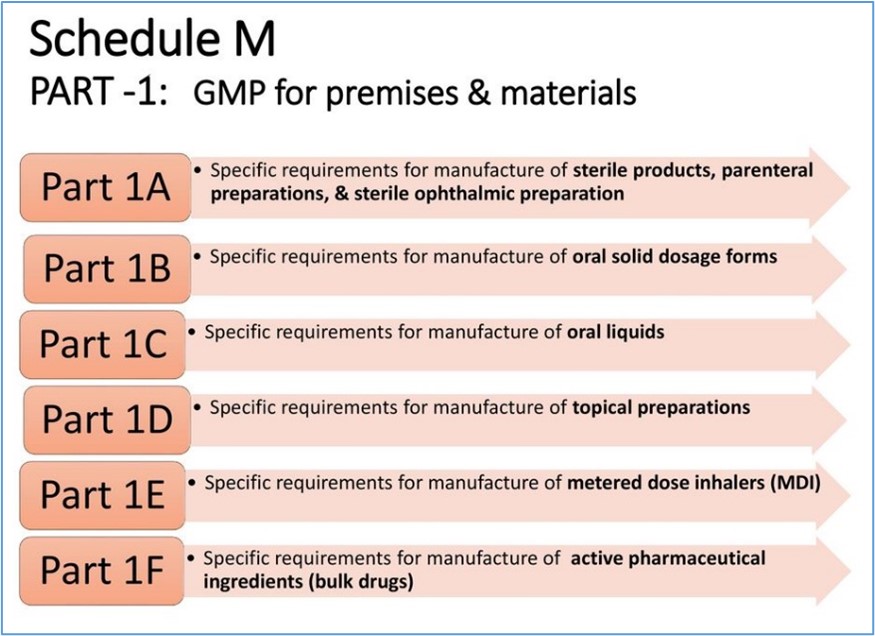
- Schedule M prescribes the Good Manufacturing Practices (GMP) for pharmaceutical products.
- The revised Schedule M has been notified as rules to ensure GMP is adhered to, and requirements of premises, plant, and equipment for pharmaceutical products.
- GMP was first incorporated in Schedule M of the Drugs and Cosmetics Rules, 1945 in the year 1988 and the last amendment was done in June, 2005.
Leave a Reply
Your Comment is awaiting moderation.


