PREVIOUS
Valneva’s Chikungunya Vaccine
November 15 , 2023
377 days
421
0
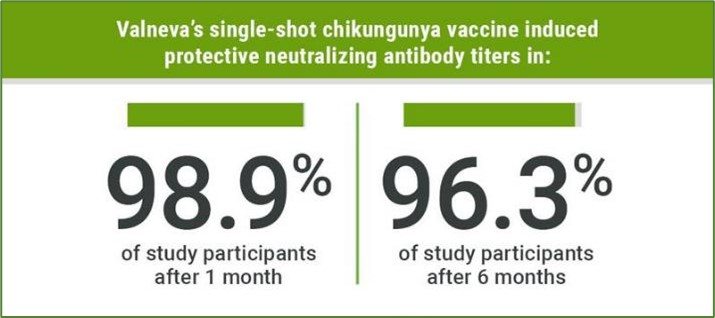
- The US Food and Drug Administration (FDA) has granted approval to Valneva's single-shot vaccine, Ixchiq.
- Now, this can be used for the prevention of chikungunya virus in adults aged 18 and older.
- This marks a major milestone as the first preventive shot approved in the United States for adults suffering from chikungunya, a mosquito-borne disease.
- Chikungunya virus is primarily transmitted to humans through the bite of infected mosquitoes.
- At least 5 million cases reported in the past 15 years.
Leave a Reply
Your Comment is awaiting moderation.


